A potential new form of pain management for your IC/BPS patients

A phase 2 clinical study from Ironwood will evaluate IW-3300, a novel, rectally administered foam, which is designed to target the inhibition of pain receptors in the colon and modify pain relief in the bladder.
For those patients who do not respond adequately to other therapies, this investigational treatment may provide an alternative to more invasive pain management options (catheterization, for example).
IW-3300 is an agonist peptide for Guanylate cyclase-C (GC-C), a receptor located primarily within the large and small intestines.
Based upon phase 1 study data, IW-3300 appears to be well tolerated.
Protocol and Study Design
|
Protocol Title |
A Phase 2 Randomized, Double-Blind, Placebo-Controlled, Adaptive Study to Evaluate the Efficacy, Safety and Tolerability of Two Dose Levels of IW-3300 Administered Rectally for 12 Weeks to Treat Bladder Pain in Subjects with IC/BPS |
|
Indication |
Patients with IC/BPS |
|
Study Drug |
IW-3300 compared with placebo |
|
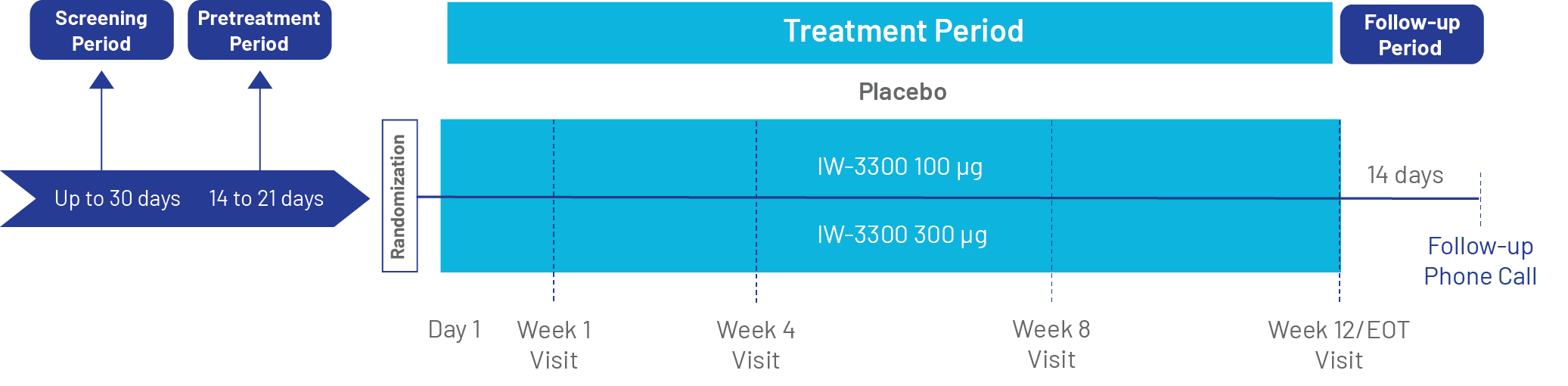
Study Design |
|
|
Study Population |
Number of patients to be randomized: 300 |
|
Treatment |
|
|
Study Centers |
Approximately 50 sites within the U.S. |
|
Cohort Groups and Doses |
|
|
Study Duration |
|
Protocol and Study Design
Objective and Endpoint
|
Primary Objective: |
Primary Endpoint at Week 12: |
|
Investigate the efficacy of IW-3300, administered as a rectal foam, on bladder pain in subjects with IC/BPS |
Change from baseline in weekly average of daily bladder pain (e.g., burning, pressure and/or discomfort) at its worst |



