
The Mahogany Clinical Trial
offers a possible treatment option to consider as an alternative for your patients with R / R FL and R / R MZL.
The investigational treatment could potentially provide them with the durable remissions and prolonged, progression-free survival they seek, with less treatment-related toxicities than other standard-of-care options.
Investigational Drug Combination
The Mahogany clinical study will evaluate the efficacy, safety and tolerability of two lenalidomide-free drug combinations that recently received fast-track approvals from the FDA. Both investigational treatments include Zanubrutinib (a BTK inhibitor) and one of two anti-CD20 monoclonal antibodies — Obinutuzumab for R / R FL, or Rituximab for R / R MZL.
Each of the three drugs comprising the investigational treatments also has been approved individually to treat other forms of lymphoma.
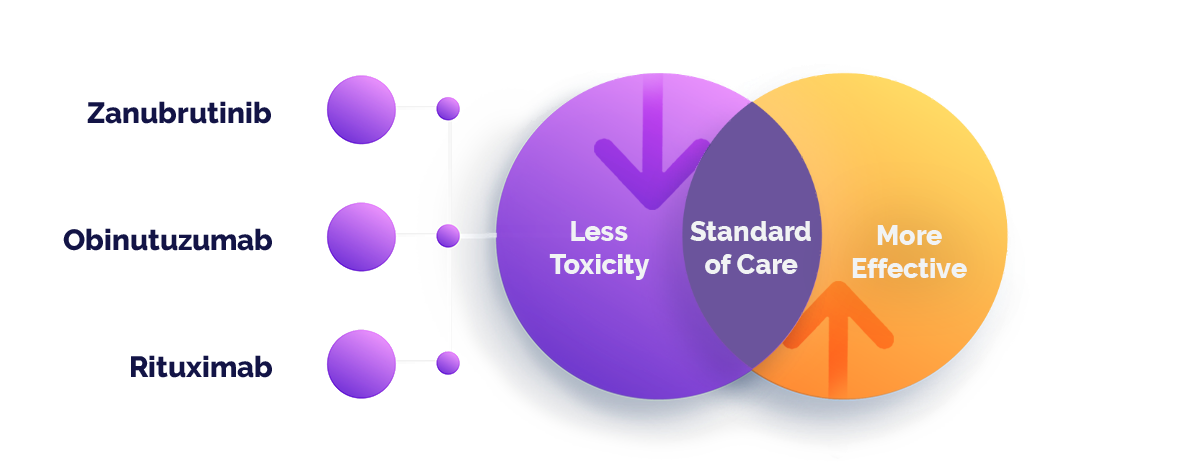
Zanubrutinib is a potent, specific and irreversible second-generation BTK inhibitor with a 50 percent maximum inhibitory concentration.
Previous studies of Zanubrutinib have shown the drug to have greater potency and selectivity than other BTK inhibitors, as well as favorable pharmacologic and toxicologic profiles. Zanubrutinib has been approved in a number of countries — including the U.S. and Canada — for patients with R / R MZL after at least one prior line of an anti-CD20-based regimen.
Obinutuzumab is an anti-CD20 monoclonal antibody.
Obinutuzumab is an anti-CD20 monoclonal antibody that provides increased direct cell-death activity and enhanced antibody-dependent cellular cytotoxicity (ADCC) activity without the complement-dependent cytotoxicity (CDC) activity associated with type I antibodies. Obinutuzumab has previously been indicated for the treatment of various B-cell malignancies including FL.
Rituximab is a genetically engineered, chimeric murine / human monoclonal kappa antibody.
Rituximab is a genetically engineered, chimeric murine / human monoclonal kappa antibody directed against the CD20 antigen, which has been previously indicated for a number of oncologic and non-oncologic treatments in various settings, including FL and indolent non-Hodgkin lymphomas.
Either the investigational drug plus the anti-CD20 antibodies or the standard of care (Lenalidomide plus Rituximab [R2]).
Close care and monitoring with experts in R / R FL and R / R MZL.
Reimbursement for study- and travel-related expenses.
Protocol and Study Design
Protocol Title
A Phase 3 Randomized, Open-Label, Multicenter Study of Zanubrutinib (BGB-3111) Plus Anti-CD20 Antibodies versus Lenalidomide Plus Rituximab in Patients with Relapsed / Refractory Follicular or Marginal Zone Lymphoma
Indication
Patients with R / R FL or R / R MZL
Investigational Drug
Zanubrutinib (BGB-3111), a BTK inhibitor, combined with an anti-CD20 monoclonal antibody (Obinutuzumab for the FL cohort; Rituximab for the MZL cohort)
Study Design
A randomized, open-label, multicenter, global Phase 3 study comparing the efficacy and safety of Zanubrutinib (BGB-3111) plus anti-CD20 monoclonal antibodies versus R2 in patients with R / R FL or R / R MZL
Study Treatment
• R / R FL Cohort: Zanubrutinib plus Obinutuzumab
• R / R MZL Cohort: Zanubrutinib plus Rituximab
Cohort Groups and Doses
R / R FL Cohort:
- Arm A:
Zanubrutinib 320 mg QD plus Obinutuzumab 1,000 mg, or
Zanubrutinib 160 mg BID plus Obinutuzumab 1,000 mg - Arm B:
Lenalidomide 20 mg QD plus Rituximab 375 mg/m2, or
Lenalidomide 10 mg QD plus Rituximab 375 mg/m2
R / R FL Cohort:
- Arm A:
Zanubrutinib 320 mg QD plus Rituximab 375 mg/m2, or
Zanubrutinib 160 mg BID plus Rituximab 375 mg/m2 - Arm B:
Lenalidomide 20 mg QD plus Rituximab 375 mg/m2, or
Lenalidomide 10 mg QD plus Rituximab 375 mg/m2
Study Duration
Screening Period:
28 days prior to initial treatment
Treatment Period:
12 months or up to progression
Follow-up Period:
up to 24 months
Primary and Secondary Objectives
Primary Objective:
- To compare the efficacy of the investigational drug versus the standard of care in patients with R / R FL and MZL
Secondary Objectives:
- To compare health-related quality of life of the investigational drug versus the standard of care based upon three patient-reported outcome questionnaires
- To compare the safety and tolerability of the investigational drug versus the standard of care

